Citric Acid Monohydrate: A Deep Dive into its Role and Evolution
Historical Development
Citric acid has roots dating back to ancient civilizations, where its tartness showed up in lemon and lime juice, long before science teased apart its structure. Researchers began isolating it around the late 18th century, when Carl Wilhelm Scheele pulled tiny crystals from lemon juice, opening the door for deeper exploration. As industrial needs grew, reliance on citrus fruit harvests proved unsustainable for large-scale supply, prompting shifts in production. The early 1900s marked a turning point after James Currie demonstrated how to use the mold Aspergillus niger to produce citric acid from sugar, sparking fermentation-based manufacturing. This approach still shapes the industry, supporting food, pharmaceutical, and cleaning sectors around the globe.
Product Overview
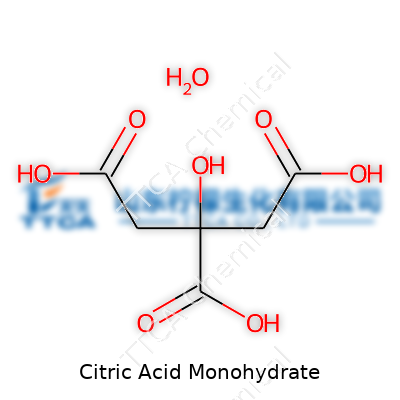
Citric acid monohydrate appears as colorless or white crystals and stands out for its sour taste. Its monohydrate form holds a single water molecule per molecule of acid, giving it slightly different handling characteristics compared to its anhydrous cousin. Found in countless consumer goods—soft drinks, candies, yogurts, and canned foods—it brings acidity, prolongs shelf life, and acts as a flavor enhancer. Manufacturers rely on it for reliable pH adjustment in foods, personal care products, and cleaning sprays. Its low price and non-toxic profile have earned its place on ingredient lists across industries.
Physical & Chemical Properties
Citric acid monohydrate sets itself apart with a melting point near 153°C, at which it loses water and shifts to its anhydrous state. Its crystals dissolve readily in water and ethanol, releasing hydrogen ions and pushing liquids towards acidity. Chemists see its chemical formula as C6H8O7·H2O. It forms chunky, odorless crystals that flow easily when dry but can clump under damp conditions. Its molecular weight sits just over 210 g/mol. Its ability to bind metal ions—chelating power—lets it trap calcium and magnesium, making it valuable for softening water in cleaning and industrial applications.
Technical Specifications & Labeling
Quality standards keep citric acid monohydrate fit for its intended uses. Specifications include minimum purity levels, usually topping 99.5% for food-grade product, and tight limits on heavy metal contaminants like arsenic or lead. Moisture content remains monitored to ensure stability, while particle size gets noted for users who need free-flowing powder in automated filling lines. Labeling regulations ask for batch numbers and manufacturing dates, plus handling instructions for shipping and storage. In the United States, it appears on labels as an approved food additive, listed by its standard name or E number E330 in the European Union.
Preparation Method
Modern production depends on submerged fermentation of carbohydrates from sources like sugar beets, corn, or cane sugar. After inoculating vats with Aspergillus niger spores, the fungi digest sugars, releasing citric acid into solution. This broth undergoes filtration to remove cells, concentration to boost yield, and precipitation with calcium hydroxide to isolate crude calcium citrate. Sulfuric acid breaks apart this intermediate and sets free citric acid, which crystallizes from water and then dries into either monohydrate or anhydrous products, depending on post-processing steps. Each phase invites strict scrutiny for microbial hygiene and chemical purity.
Chemical Reactions & Modifications
Citric acid’s three carboxylic acid groups give it a knack for reacting with bases and alcohols, leading to salts and esters that find use in different markets. When reacted with sodium or potassium hydroxide, it forms sodium or potassium citrate, crucial for medicines and buffering agents. Reacting with alcohol sets off esterification, producing triethyl citrate, a plasticizer for food wraps and cosmetics. In cleaning, its chelating ability teams up with oxidizers to help remove stubborn deposits and rust. Chemists tweak its structure for specific niches, sometimes crosslinking it for specialized polymers or pairing it with surfactants for advanced detergent blends.
Synonyms & Product Names
Citric acid monohydrate travels under several names. Labels cite it as 2-hydroxy-1,2,3-propanetricarboxylic acid monohydrate, E330, Lemon Salt, or just plain Citric Acid Monohydrate. Product codes from major suppliers may distinguish between food, pharma, or technical grades, but the core substance remains consistent. Scientific circles recognize its IUPAC name, giving clarity in research or regulatory paperwork.
Safety & Operational Standards
Manufacturers operate under frameworks like ISO 9001 and HACCP, guiding sanitation and traceability through every step. Though citric acid monohydrate ranks as low hazard, dust may irritate airways or skin with frequent handling, and workers wear gloves or masks in production zones. The FDA classifies it as generally recognized as safe (GRAS), and it passes muster for both Kosher and Halal certifications. Storage conditions favor cool, dry places, in sealed packaging to keep out moisture. Transportation by road or sea meets strict labeling and spill preparedness requirements to protect both handlers and the environment.
Application Area
Few ingredients match the versatility of citric acid monohydrate. In foods, it drops pH just enough to keep microbes at bay, perks up citrus flavors, and stabilizes color in jams, jellies, soft drinks, and more. Pharmaceutical makers reach for it to balance pH in oral medications, help dissolve active ingredients into syrups, and provide effervescence in fizzy tablets. Cosmetics makers turn to its play between acidic and alkaline for face washes, shampoo, and even bath bombs. Cleaning and water treatment rely on its chelating edge, scrubbing away mineral scale in kettles, boilers, and dishwashers. Even photographers and winemakers trust it to correct pH and tame reactive metals in their crafts.
Research & Development
Labs keep stretching what citric acid monohydrate can do. Biotechnologists tinker with microbial strains for better yields or cleaner fermentation byproducts, racing to lower production costs. Materials scientists fold citric acid derivatives into edible films for packaging or biocompatible medical patches, exploring options for greener consumer goods. Green chemistry looks at recycling food waste as a fermentation feedstock, chipping away at the industry’s carbon footprint. Analytical chemists publish new ways to monitor trace contaminants, extending the ingredient’s reach into new regulatory zones and high-purity markets.
Toxicity Research
Centuries of use give citric acid monohydrate a strong record for safety. Animal studies point to low acute toxicity, even at high doses, and it passes rapidly through the body via standard metabolic routes. Eating or drinking small to moderate amounts rarely brings strong side effects outside dental erosion with habitual overuse. Those with rare metabolic disorders such as citric acid cycle enzyme deficiencies need to steer clear. Direct eye contact stings, and inhaled dust may aggravate asthma in rare cases. Government watchdogs like the EPA and EFSA regularly revisit safety profiles to confirm these findings and update exposure limits if needed.
Future Prospects
Citric acid monohydrate stands at a crossroads shaped by sustainability, public health, and the ongoing search for clean-label ingredients. Manufacturers look for ways to swap fossil-based feedstocks with agricultural byproducts, leaving less landfill waste behind. As the public grows wary of artificial additives, food formulators lean into citric acid’s natural roots, adding marketing value along with flavor. Water scarcity and tighter regulations push cleaning product makers toward biodegradable, safe ingredients, opening more doors for citric acid solutions. In personal care, formulators try new blends that gently adjust pH without stripping skin, keeping up with demand for sensitive, eco-friendly products. Academic and industry partnerships keep pushing, from engineered microbes to more efficient application methods, ensuring citric acid remains a mainstay in years to come.
More Than Just a Food Ingredient
Citric acid monohydrate sounds mighty scientific, but you'll bump into it in places that might surprise you. I've spent years reading labels, cooking at home, and even mixing up home cleaning supplies. This ingredient shows up on everything from snack wrappers to cleaning bottles. It's not some mysterious additive from a lab; it's a natural acid found in citrus fruits, made convenient for all kinds of uses. Sure, its most familiar job is making food and drinks taste tart or refreshing. Without it, soft drinks lack that sharp edge people expect. Even candies would taste flat. The reason it gets the nod over plain lemon juice comes down to consistency—brands know exactly how sour each batch turns out. Bakers use it in cakes and jams for the same effect, and it keeps fruits looking fresh instead of brown and dull.
Keeping Food Safe and Fresh
Food goes bad fast. Nobody wants to bite into an apple slice and find it two shades browner than when it was cut. Citric acid monohydrate solves this in commercial kitchens and at home. Its acidity slows down the gunk that spoils fruit and even slows mold. The cheese in your fridge or canned tomatoes on the pantry shelf? There’s a good chance citric acid played a part in making those possible and lasting just a little longer. And, unlike some synthetic preservatives, this stuff comes from plant sources, so most folks feel comfortable with it in their diet.
Cleaner Living With Less Fuss
Some days, I look for cleaning products without harsh chemicals. Citric acid monohydrate cuts through hard water stains and limescale almost like magic, and you won't have to worry about toxic fumes. In old coffee makers or kettles, it dissolves mineral build-up in ways soap can't match. Plenty of companies producing eco-friendly cleaners replace strong bleach with citric acid. It’s safe to touch and rinse away, which is helpful if you have kids or pets in the kitchen.
An Important Player in Medicine and Beauty
Chemistry plays a huge part in making medicines safe and stable. Pharmacies and drug companies use citric acid monohydrate to balance pH and keep medicines from spoiling. Vitamin C tablets often list this as part of their makeup. Toothpastes, mouthwashes, and even shampoos use it to balance acidity so products work well and feel good.
Challenges and Safer Choices
Though useful, high amounts can upset sensitive stomachs or contribute to gum irritation, especially when found in sour candies or drinks. It’s not a health risk for most people at normal levels, but anyone with allergies or chronic digestive problems might feel otherwise. Some avoid it for peace of mind, choosing foods with less-added stuff. Producers can address this by labeling ingredients clearly, offering options that use alternative acids, or reducing total amounts in products aimed at kids and sensitive eaters.
Finding the Balance
Citric acid monohydrate stands out for simplicity, safety, and versatility, but it’s just one piece of what goes into food, cleaners, and health products. By learning what’s inside the things we use and eat, everyone gets a better shot at making choices that match their own needs and values.
What Citric Acid Monohydrate Actually Is
Open any pantry, and chances are you’ll spot citric acid on the label of something. It’s common in soft drinks, jams, packaged foods, and even some dietary supplements. Citric acid monohydrate is just citric acid with a small amount of water attached to its structure – a natural compound found in citrus fruits like lemons and limes. In the food industry, most of it comes from a fermentation process involving tiny fungi such as Aspergillus niger. This process has been around for decades, allowing the world to meet the high demand for tart flavors and reliable food preservation.
The Science Behind the Safety
Many people ask questions about the safety of citric acid monohydrate. That’s wise in an age where ingredient names look less like something from grandma’s kitchen and more like chemistry class. Fortunately, food safety agencies such as the U.S. Food and Drug Administration and the European Food Safety Authority have given citric acid the green light. They consider it “generally recognized as safe” (GRAS). Years of research and everyday consumption haven’t raised any major red flags for most folks.
Citric acid plays a role in your body’s basic energy cycles. Your cells actually rely on natural citric acid every day to create energy from the food you eat. So there’s a familiarity your body already has with this compound.
What Real-Life Experiences Show
Most people swallow products with citric acid nearly every day with no issue. I’ve been reading ingredient lists for over a decade, and it’s clear that the bulk of people don’t notice any difference from choosing foods with citric acid as a preservative or flavor booster. Hospitals and emergency rooms aren’t reporting sudden spikes in problems related to it. There are exceptions, though. A small number of people with allergies or rare medical conditions like citric acid intolerance could react to high amounts. They might experience stomach discomfort or mouth sores. Usually, these cases are rare and often linked to large or repeated ingestion.
Why Are People Worried?
The internet hosts a huge wave of anecdotes. Some worry citric acid from industrial sources might harbor traces from the fungi used in production. But regulatory agencies set strict standards for purity. Every batch gets tested for contaminants, and food manufacturers rely on certifications and audits to catch any issues long before food hits store shelves.
People sometimes blame food acids for dental erosion too. Acidic drinks and candies can wear down enamel if you sip or snack constantly. That’s a real concern, but it points more to how often acidic products are consumed, not just the presence of citric acid itself.
Building Trust and Better Choices
Transparency matters. Ingredient lists should be easy to understand, and food makers should keep lines open with consumers. If someone has a rare sensitivity, it’s good practice for doctors and nutritionists to help navigate label language. Parents with kids facing allergies or people with sensitive digestion might want to jot down which foods brought on symptoms.
Balancing a varied diet helps, too. Fresh fruits, vegetables, and whole grains allow anyone to avoid the need for high intake of manufactured additives. Moderation, good dental hygiene, and education let most people enjoy foods with citric acid monohydrate safely. Food safety boils down to solid science, common sense, and honest conversations between industry, experts, and the public.
Everyday Encounters With Citric Acid
Walk into any kitchen or grocery store, and citric acid shows up sooner than you’d think. It adds a sharp kick to sodas, preserves fruit, and brings balance to a sauce. For years, I kept a small jar of it on my spice shelf for home canning. You don’t need to run a lab to see how often citric acid works its way into daily routines, whether as a tangy punch in lemonade or a cleaner for the coffee pot.
Some folks notice another name on ingredient lists: citric acid monohydrate. This sounds technical, but both come from the same source—citrus fruit or microbial fermentation. The difference comes down to water. Regular citric acid, often called anhydrous, means all its water got removed during processing. Monohydrate holds on to a single water molecule per molecule of citric acid, locked in by its crystalline structure.
Where the Difference Matters
Bakers, brewers, and food manufacturers care deeply about these distinctions. That extra water in monohydrate means a subtle difference in weight. If you swap one gram of anhydrous citric acid for one gram of monohydrate, the recipe’s tartness might drop just a little. For someone selling jams under strict FDA labeling rules or formulating sports drinks, that minor water content makes all the difference.
In kitchens at home, this often goes unnoticed. Most recipes with “citric acid” mean either form works, though pure flavor fanatics or chefs committed to precision—sour candies come to mind—might look up the exact amount to adjust a recipe. In pharmaceuticals, manufacturers rely on these details. Tablets dissolve faster or slower based on water inside the ingredient. Stored in a humid basement, monohydrate clumps less or more, which means shelf-life matters.
Personal experience running a tiny natural cleaning business taught me that both types tackle limescale and soap scum effectively. But differences show up when mixing batches for shelf storage. Anhydrous chunks together in damp air faster, sometimes turning my powder into a stubborn brick. A bit of knowledge here saves money and effort, as small shops can pick the version that fits their humidity.
Safety, Sourcing, and Trust
Citric acid—no matter the form—has earned its place as a safe, trusted staple food acid, approved by the FDA and worldwide regulators. Decades of studies back up its use in food and cleaning. Still, headlines pop up from time to time about synthetic versus “natural” citric acid. Over 90% of today’s supply comes from fermenting simple sugars with mold. This process, started over a hundred years ago, creates food-grade powder. Sourcing matters for some folks with allergies, and clarity on supply chain can help them feel safe in their choices.
Scientific research supports both anhydrous and monohydrate forms as safe for human use in recommended doses. Neither is tied to the kinds of chemical additives that often cause alarm. Diligence matters, especially for anyone mixing their own products or with unique health concerns. Reading the supplier’s verification and choosing reliable brands keeps risk low.
Finding Solutions in Practice
Labels could serve everyone better. Instead of just “citric acid,” jars, boxes, and food packages might say “anhydrous” or “monohydrate.” For consumers and hobbyists, having this small piece of information could keep food tastier and home experiments more predictable. For manufacturers, investing in clear employee training on the differences avoids costly mix-ups.
People want to trust what’s going into their bodies and homes. Industry and regulators should work together to keep supply chains transparent, dig into research updates, and share straight talk about ingredient forms. Home cooks, makers, and anyone curious about what they’re eating deserve the facts. That keeps trust alive and the food we love both safe and delicious.
Why Storage Choices Matter
Walk into any pharmacy, bakery, laboratory, or even a home pantry, and you’re likely to find a container of citric acid monohydrate. People rely on it for sour candies, bath bombs, and buffering solutions. If you look past the label, though, proper storage becomes more than a recommendation—it’s the line between useful, safe material and spoiled product. From my work in food production and some time handling chemicals in university labs, I learned this lesson the hard way: the way you store things can save you money, time, and effort, or create a heap of unnecessary problems.
Keep Moisture Out
Citric acid monohydrate draws water from the air, turning hard and lumpy within weeks if left open. Humidity sneaks in quickly, especially in kitchens or storerooms near sinks and pipes. I’ve seen what happens when even a loosely closed bag sits near a steaming kettle. The powder becomes a useless block. To avoid this, always use airtight containers, preferably ones made of glass or thick plastic with strong seals. If the facility is busy and containers get opened often, tossing in a food-safe desiccant packs some extra insurance against dampness.
Stay Away from Heat and Light
Excess heat shortens the lifespan of citric acid monohydrate. Under warm conditions, the compound might lose its waters of crystallization. This changes its weight and performance for recipes or processes relying on precise chemistry. I always store powdered ingredients below chest height on the coolest side of a storeroom, well away from ovens, radiators, and sunny window ledges. If direct sunlight hits the storage site, chemical stability drops—and you may not notice for months, until half the batch disappoints you at crunch time.
Avoid Contamination by Chemicals or Odors
Citric acid pulls in odors just as it draws moisture. One time, I stored a bag alongside an open bottle of vinegar—and wound up with sour-tasting bath products that nobody wanted to use. Keep your supply well away from volatile chemicals, cleaning agents, strong spices, and other reactive substances. Using clearly labeled, dedicated scoops stops people from slipping up and accidentally mixing other powders in. In shared storage environments, reminding staff or family members about the rules saves grief later.
Other Storage Tips Learned through Experience
Purchase citric acid monohydrate in a volume that matches how quickly you use it. Large open bags might look thrifty, but if you struggle to reseal them, you’re risking waste. Break bulk supplies into smaller, sealed containers for daily use. Remember to label everything with the purchase date and watch for changes in color or texture—these warn you of contamination or degradation.
Finally, check your local regulations and supplier advice, especially if you handle citric acid for resale or in food production. Good storage habits reflect care, not just for the product, but for everyone using it down the line.
What Is Citric Acid Monohydrate?
Citric acid monohydrate comes from citrus fruits, but it shows up in kitchens, factories, and supermarkets more often as a white, crystalline powder. This form of citric acid keeps a small amount of water, which helps its stability during storage. You’ll spot it on food labels—usually just called “citric acid.” Squeezing a lemon for zing is one thing, but large food companies need volume, consistency, and purity. They rely on this ingredient for a reason.
Why Do Companies Use It?
People have enjoyed tart flavors since ancient times, but mass food production brought new challenges. Preserving food, making drinks pop with flavor, and keeping color bright all matter more now. Citric acid monohydrate manages these jobs efficiently. Added to sodas and candies, it creates that unmistakable sourness. In jams, jellies, or canned tomatoes, it lowers pH, which helps push out spoilage-causing bacteria. Its use keeps food safer for longer, which is no small thing in a world where food waste piles up and recalls can cost a company millions.
Is It Safe to Eat?
Chemical-sounding names sometimes scare people, but science backs citric acid’s safety. The U.S. Food and Drug Administration considers it “Generally Recognized As Safe.” The World Health Organization reviewed its effects as well. Citric acid exists naturally in many fruits—it’s only when manufacturing scales up that the name sounds unfamiliar. Even at high doses, harmful effects only show up in rare situations. The most likely side effect is mild stomach upset if someone goes overboard eating sour candies.
Potential Drawbacks
Packing lots of sour candies or acidic drinks into a diet can harm tooth enamel. I remember eating mouth puckering warheads as a kid; my dentist was less enthusiastic about my sweet-and-sour habit. The acid levels in drinks like sodas can weaken enamel over time. Drinking through a straw and rinsing with water afterward limits this damage, according to the American Dental Association. Some people also report mild mouth or digestive irritation from products with added acid, but most folks don’t notice any problems at all.
Where Is It Found?
Citric acid monohydrate appears in all sorts of foods: sodas, powdered drink mixes, canned vegetables, candies, jams, dairy, and even bread. Its popularity comes down to being reliable and affordable. Unlike fruit juice, it tastes consistent and doesn’t spoil easily. Bakers might sprinkle it in dough for tartness, while chefs use it for balancing flavors in sauces.
Improving Use and Addressing Concerns
The real opportunity for improvement lies in consumer education and transparency. Labels sometimes feel hard to understand. Food companies that explain why ingredients like citric acid are there build trust. People deserve to know how these ingredients benefit freshness and safety. For those who watch out for additives, support for better labeling and simple customer communication matters. At home, moderation is key—the usual advice for sugar or salt holds true for acidic ingredients as well.
Final Thoughts
Citric acid monohydrate offers more than just a sharp flavor; it carries food traditions forward, supports safer processing, and supports the search for less waste. With sound science and honest conversation, it continues to play a helpful part in modern food and drink.